Structure and catalytic properties of novel copper isatin Schiff base complexes†
Abstract
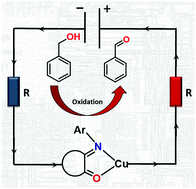
Isatin Schiff base ligands in combination with redox active metal ions have the potential to behave as non-innocent ligands facilitating industrially important chemical reactions. The ligand structure is easily modified by introducing substituents in three different positions, affecting the electron density distribution that was evaluated by Mulliken charge analysis and cyclic voltammetry for a range of isatin derivatives. It was noticed that coordination of these ligands to copper(II) bromide in alcohol resulted in copper reduction to Cu(I) species and alcohol oxidation. Compared to organic chemistry, the inorganic chemistry of these ligands remains poorly examined. Here, we present the structural study of sixteen novel copper complexes with mononuclear [Cu(L)2]Hal2/1 and halo-bridged binuclear [Cu2(μ-Hal)2(L)2] structures (L = isatin Schiff base ligand; Hal = Cl, Br and I). Finally, application of the above complexes for alcohol oxidation was evaluated: the complexes selectively catalyse benzyl alcohol oxidation to the corresponding aldehyde with almost quantitative yields and high selectivity.


 Please wait while we load your content...
Please wait while we load your content...