Ultrasonication-assisted synthesis of ternary-component Ni3AlxFe1−x-layered double hydroxide nanoparticles for the oxygen evolution reaction in a neutral solution
Abstract
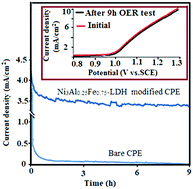
In this study, ultrasonication, a facile and rapid process, was utilized for the preparation of ternary-component layered double hydroxides (LDHs), Ni3AlxFe1−x-LDHs, as the electrocatalyst material for the oxygen evolution reaction (OER) in a neutral solution. Despite the fact that bivalent metal ions, such as Ni2+, Co2+ and Zn2+, have been demonstrated to play a highly specialized role in water oxidation or OER, the role of trivalent metal ions in LDH structures within these areas requires a lot of research. Hence, herein, we demonstrated that Al3+ had a positive effect on the size and the bandgap energy of the as-synthesized LDHs, which led to the improvement in electrochemical performance. According to powder X-ray diffraction and field-emission scanning electron microscopy, it was found that Al3+ ions could regulate the crystallinity and the size of Ni3AlxFe1−x-LDHs nanoparticles. Ni3AlxFe1−x-LDHs were used as electrocatalyst materials in carbon paste electrodes (CPEs) for the OER in a neutral solution. The obtained results showed that the electrocatalytic activity of Ni3Al0.25Fe0.75-LDH was much better than the other binary-component LDHs. The Tafel slope of the OER in 0.1 M phosphate buffer (pH = 7) at the ternary-component Ni3Al0.25Fe0.75-LDH electrocatalyst (15 mV dec−1) was lower than that of Ni3Fe-LDH (61 mV dec−1) and Ni3Al-LDH (98 mV dec−1); also, it exhibited a low overpotential for this process. The high electrocatalytic activity of Ni3Al0.25Fe0.75-LDH may be attributed to the co-existence of Al3+ and Fe3+ active sites and also to its decreased bandgap in comparison to that of Ni3Fe-LDH and Ni3Al-LDH.


 Please wait while we load your content...
Please wait while we load your content...