A mixed phenoxo and end-on azide bridged dinuclear copper(ii) Schiff base complex: synthesis, structure, magnetic characterization and DFT study†
Abstract
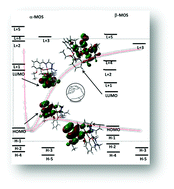
A dinuclear copper(II) complex, [(H2O)Cu(L)(μ1,1-N3)Cu(L)]ClO4 [HL = (2-(2-(ethylamino)ethylimino)methyl)-6-ethoxyphenol] has been synthesized and characterized by spectral and elemental analyses. The structure has been confirmed by single crystal X-ray diffraction. Variable temperature (2–300 K) magnetic susceptibility measurements indicate the presence of antiferromagnetic exchange coupling between the copper(II) centers (J = −46.18 cm−1), as also corroborated by DFT calculations (Jtheo = −45.98 cm−1). The calculated spin densities at the copper(II) centers were +0.56 and −0.61, confirming that they are the magnetic centers. An experimental absorption spectrum of the complex has been compared with a normalized absorption spectrum obtained theoretically. Supramolecular interactions in the solid state of the complex have also been characterized with the help of Hirshfeld analysis and the AIM theory.


 Please wait while we load your content...
Please wait while we load your content...