Design, synthesis, and phase behaviors of a novel triphenylene-based side chain liquid crystalline diblock copolymer
Abstract
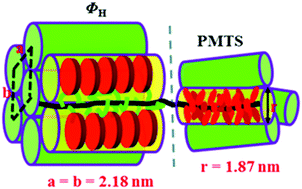
A novel double Tp-based liquid crystalline (LC) diblock copolymer (PMTS-b-PMT6S) composed of poly[3,6,7,10,11-pentakis (hexyloxy)-2-oxytriphenylene] (PMTS) and poly{6-[3,6,7,10,11-pentakis(hexyloxy)-2-oxytriphenylene]} (PMT6S) was designed and successfully synthesized by reversible addition–fragmentation chain transfer (RAFT) polymerization. While PMTS is a rigid columnar-shaped (ΦN) polymer, PMT6S is a stable hexagonal columnar phase (ΦH) polymer. The phase behaviors of diblock copolymers were studied by DSC, POM and 1D WAXD. The results showed that the weight fraction of PMT6S (fPMT6S) has a significant effect on the LC phase behaviors and phase structures of diblock copolymers. Both glass transition temperature and phase transition temperature of the diblock copolymers from LC phase to isotropic phase reduced with the weight fraction of PMT6S in the feed. When the fPMT6S ≤ 58.1%, PMTS-b-PMT6S-1 to PMTS-b-PMT6S-3 show similar properties to PMTS, which formed a stable columnar nematic phase (ΦN), while when the fPMT6S ≥ 64.2%, PMTS-b-PMT6S-4 and PMTS-b-PMT6S-5 show similar properties to PMT6S, which presented a hexagonal symmetry columnar phase (ΦH). Comparison between the diblock copolymer and homopolymer (PMTS and PMT6S) indicates that the content of the spacer was crucial to determine the LC structures. Through the study one can better understand the interrelation of microstructures and Tp DLC orders, which constitutes the key basis for various applications.


 Please wait while we load your content...
Please wait while we load your content...