Resorcylic acid lactones (RALs) and their structural congeners: recent advances in their biosynthesis, chemical synthesis and biology
Abstract
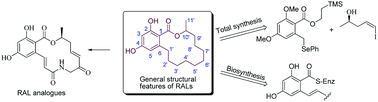
Resorcylic acid lactones (RALs) are naturally occurring 14-membered macrolactones that constitute a class of polyketides derived from fungal metabolites and that possess significant and promising biological activity. Their core structural feature consists of a β-resorcylic acid framework (2,4-dihydroxybenzoic acid) fused with an alicyclic side unit decorated with numerous functional groups in a stereodefined fashion. In this review, we focus our attention on the chemistry and biology of this novel class of macrolactones. Only recent developments from the year 2008 to date will be covered, and the core attention will be given to the synthesis and biosynthesis of RALs published during these times. We also delineate the chemistry and biology of several structural congeners of RALs that have also come into existence in recent years.


 Please wait while we load your content...
Please wait while we load your content...