Electrochemical synthesis of some 2-aminobenzofuran-3-carbonitrile and 2-aminobenzofuran-3-carboxylate derivatives: product diversity by changing the applied current density†
Abstract
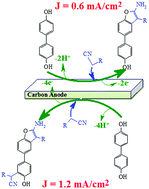
Green syntheses of two different series of new benzofuran derivatives were carried out by anodic oxidation of 4,4′-biphenol (4BP) in the presence of some CH-acid compounds (malononitrile, methyl cyanoacetate and ethyl cyanoacetate) as nucleophiles by controlling the potential during electrolysis. The results indicate that the CH-acid compounds participate in a Michael-like addition reaction with the electrochemically generated diphenyl-p-quinone (DPQ), converting it to the corresponding products depending on the applied electrode potential or current density. In this study, the desired products in a water/ethanol (50/50, v/v) mixture were obtained with high yield and high atom economy using a simple and reagentless green galvanostatic method.


 Please wait while we load your content...
Please wait while we load your content...