Highly emissive host–guest based on nanoclay intercalated with an Eu3+ complex bearing a new Ru2+ organometallic ligand†
Abstract
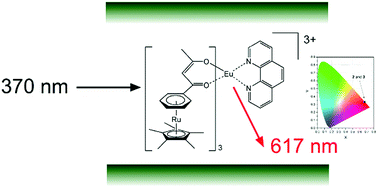
This work describes the design, synthesis and physicochemical properties of the highly emissive nanocomposite [bentonite][Eu{(η5-Cp*)Ru(η6-bzac)}3Phen]0.09[H2O]0.22 based on the Eu(III) complex [Eu{(η5-Cp*)Ru(η6-bzac)}3Phen][PF6]3, bearing a new Ru(II) organometallic β-diketone ligand [(η5-Cp*)Ru(η6-Hbzac)][PF6], intercalated into bentonite-Na+ nanoclay. Single crystal X-ray diffraction analysis of the ligand showed that the [(η5-Cp*)Ru]+ is bound to the phenyl group of a highly planar and conjugated organic β-diketone 1-phenyl-1,3-butanedione (Hbzac) through η6-coordination. The complex was inserted into bentonite-Na+ by ion exchange reactions at room temperature. The products display interlaminar distances and stoichiometries in agreement with the ion exchange capacity and the interlayer space available in the clay. Thermal gravimetric analysis was used to establish the decomposition temperatures of the complex and the nanocomposite, which exceed or border 280 °C. Both compounds exhibit luminescence of high colorimetric purity in the red region. The intensity of the characteristic narrow emission band of Eu3+ in the red region, corresponding to 5D0 → 7F2 transition, is accentuated in the intercalated nanocomposite, reaching a symmetry parameter R = I(5D0 → 7F2)/I(5D0 → 7F1) and Judd–Ofelt Ω2 parameter approximately three and two times larger respectively than that of the starting complex. The ruthenium(II)–europium(III) based nanocomposite is presented as a potential luminescent material with considerably high stability over time.


 Please wait while we load your content...
Please wait while we load your content...