Ion exchange for synthesis of porous CuxO/SnO2/ZnSnO3 microboxes as a high-performance lithium-ion battery anode†
Abstract
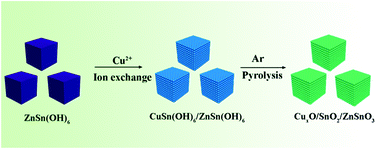
Binary Sn-based metal oxides are potential anode materials for lithium ion batteries (LIBs). However, they suffer from large volume expansion during the discharge/charge process, leading to poor cycling performance. In this work, a multicomponent CuxO/SnO2/ZnSnO3 composite with a porous structure was prepared by employing ZnSn(OH)6 microcubes as precursors with the ion exchange method. Under high temperature, Cu2+ can exchange with partial Zn2+ in ZnSn(OH)6 to form CuSn(OH)6 microboxes. Then, through an annealing process, the porous CuxO/SnO2/ZnSnO3 composite was obtained, which also retained the box morphology. The CuxO/SnO2/ZnSnO3 microboxes can deliver an improved capacity of 615 mA h g−1 due to the porous structure, which may enhance the transportation of Li+ and electrons. The ion exchange method for obtaining porous binary metal oxide nanomaterials can be used for fabricating other metal oxide composites in many areas such as LIBs, catalysts, etc.


 Please wait while we load your content...
Please wait while we load your content...