Anti-migration and burning rate catalytic performances of novel ferrocene-based porphyrins and their transition-metal complexes†
Abstract
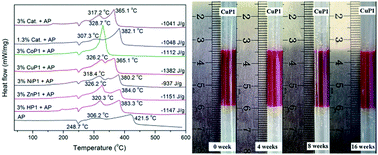
Two series of novel ferrocene-based porphyrins and their transition metal (Zn, Co, Ni, and Cu) complexes were synthesized and characterized. One has ferrocene on the skirt of meso-tetraphenylporphyrin (TPP) via long chain alkoxy groups (HP1 and HP2); the other has a close connection of TPP and ferrocene containing long chain alkyl groups by ester linkage (HP3). Their UV-visible absorption spectra, fluorescence spectra, thermal stability and electrochemical properties were investigated. They were applied for the first time as burning rate catalysts (BRCs) in a simulative solid propellant to overcome the migration problem. Their catalytic effects on the thermal degradation of ammonium perchlorate (AP) were evaluated by thermogravimetric and differential scanning calorimetry techniques, and the results showed that the catalytic effects of free porphyrin, Zn(II) porphyrin and Ni(II) porphyrin were similar to that of catocene. While Cu(II) and Co(II) porphyrins exhibited higher catalytic effects than catocene, the Co(II) complexes lowered the thermal decomposition temperature of AP more dramatically compared with Cu(II) complexes. What's more, these compounds displayed non-migratory ability in the migration test. Therefore, the novel ferrocene-based porphyrin derivatives are non-migratory BRCs with high catalytic efficiency for composite solid propellants and have potential applications in aerospace.


 Please wait while we load your content...
Please wait while we load your content...