Brønsted acid catalysed eco friendly synthesis of quaternary centred C-3 functionalized oxindole derivatives†
Abstract
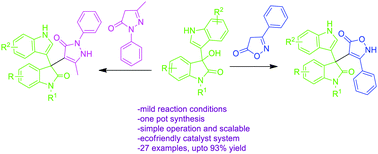
A facile atom economic and eco friendly protocol for the synthesis of biologically important quaternary centered C-3 functionalized oxindole derivatives, with a novel nucleus, in high yields has been demonstrated by employing 3-hydroxy-2-oxindole, isoxazolone/pyrazolone and environmentally benevolent p-toluene sulphonic acid as a catalyst. The advantages of this protocol are the wide substrate scope, practical simplicity, benign solvent and good yields. All the synthesized compounds were evaluated for their in vitro anti-microbial activity. Several compounds exhibited good activities comparable to those of established standard drugs. Furthermore, the anti-cancer activity of compounds 3g and 3m has been preliminarly demonstrated by in vitro evaluation against human tumor cell lines MCF-7 and Hep-2, using MTT-based assays with commercially available standard drug cisplatin as a positive control. Gratifyingly, compounds 3g and 3m exhibited good in vitro inhibitory activities against Hep-2 and MCF-7 cell lines. These results indicate that 3-indolyl oxindole substituted isoxazole analogs may be potential lead compounds for further biological screening.


 Please wait while we load your content...
Please wait while we load your content...