Synthesis, characterization and electrochemistry of polycyclic fused aromatic pyrroles and their conjugated polymers†
Abstract
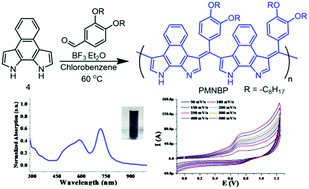
Polycyclic fused aromatic pyrrole-based compounds, benzodipyrrole, naphthobipyrrole and their alkylated derivatives, were synthesized and studied electrochemically. Amongst these, naphthobipyrrole having a tetracyclic fused aromatic structure was electrochemically polymerized to give the homopolymer of naphthobipyrrole, poly(naphthobipyrrole), indicating good reactivity of α,α′-pyrrolic positions towards oxidative coupling. As a strategic approach towards the synthesis of copolymers, naphthobipyrrole was copolymerized with arylaldehyde to afford poly(arylmethylene naphthobipyrrole) by exploring the reactivity of β,β′-pyrrolic positions. Moreover, optical and electrochemical studies showed that the copolymer possessed a low optical bandgap and an elevated HOMO level. The scan rate dependent cyclic voltammetry studies of the copolymer film showed the formation of an electroactive material.


 Please wait while we load your content...
Please wait while we load your content...