Synthesis of new spiro pyrrole/pyrrolizine/thiazole derivatives via (3+2) cycloaddition reactions†
Abstract
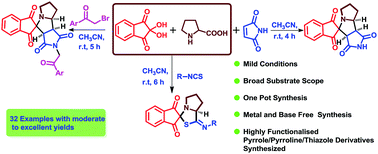
A new series of spiro pyrrole/pyrrolizine/thiazole compounds have been synthesized using (3+2) dipolar cycloaddition via three-component condensation reactions of ninhydrin and α-amino acids with different dipolarophiles such as maleimide, malic anhydride, 2-benzyl-2-methylcyclopent-4-ene-1,3-dione and isothiocyanates. Another four-component protocol has been developed in which ninhydrin, proline, maleimide and phenacyl bromides are reacted to afford a novel library of pyrrolizine derivatives in good yields without the use of catalyst.


 Please wait while we load your content...
Please wait while we load your content...