Room-temperature successive ion transfer chemical synthesis and the efficient acetone gas sensor and electrochemical energy storage applications of Bi2O3 nanostructures
Abstract
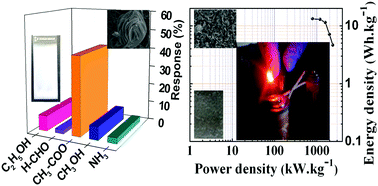
The acetone gas sensor and electrochemical supercapacitor applications of bismuth oxide (Bi2O3) nanostructures, synthesised using a facile and cost-effective quaternary-beaker mediated successive ion transfer wet chemical method and deposited onto soda-lime-glass (SLG) and Ni-foam substrates, respectively, are explored. The as-deposited Bi2O3 nanostructures on these substrates exhibit polycrystalline nature and a slight change in their surface appearance (i.e. upright-standing nanoplates on SLG and a curvy nanosheet structure on Ni-foam), suggesting the importance of the deposition substrate in developing Bi2O3 morphologies. The Bi2O3 nanoplate gas sensor on the SGL demonstrated a room temperature sensitivity of 41%@100 ppm for acetone gas, whereas the nanosheet structure of Bi2O3 on the Ni-foam elucidated a specific capacitance of 402 F g−1 at 2 mA cm−2, long-term cyclability, and rate capability with moderate chemical and environmental stability in a 6 M KOH electrolyte solution. The Bi2O3//graphite pencil-type asymmetric supercapacitor device revealed a specific capacitance as high as 43 F g−1, and an energy density of 13 W h kg−1 at 793 W kg−1 power density, turning a light emitting diode ON, with considerable full-brightness light intensity, during the process of discharging.


 Please wait while we load your content...
Please wait while we load your content...