A 3D metal–organic framework with dual-aerial-octahedral trinucleate building units: synthesis, structure and fluorescence sensing properties†
Abstract
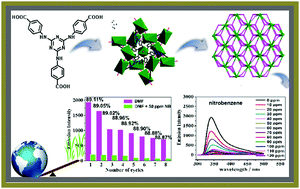
A six-coordinated 3D metal–organic framework (MOF), [ZnK(tatab)(CH3OH)]·3DMF·4.5CH3OH (JLNU-2, JLNU = Jilin Normal University, H3tatab = 4,4′,4′′-((1,3,5-triazine-2,4,6-triyl)tris(azanediyl)) tribenzoic acid, DMF = N,N-dimethylformamide), has been purposefully constructed by combining the N-donor ligand H3tatab with aromatic π rings and d10 configuration metal Zn(II) nitrate under solvothermal conditions. The crystal structure reveals that JLNU-2 consists of a dual-aerial-octahedral trinuclear potassium cluster and zinc building units with N–H⋯O hydrogen bonding, which are further bridged by N-donor multicarboxylate ligands to produce a (3,6)-connected topological net in an interesting hot-wheel state. The special hydrogen bonding strongly promotes the molecular luminescence sensitivity. The experimental results show that JLNU-2 exhibits outstanding fluorescence quenching properties via an electron transfer mechanism from the electron-rich framework to the electron-withdrawing group of nitrobenzene. The fluorescence was quenched completely in the presence of 130 ppm of nitrobenzene. After 8 cycles, the quenching efficiency was maintained at 88.9%. As a result, the MOF material is extremely well qualified to be a fluorescence sensor for the detection of nitrobenzene owing to its supreme selectivity, sensitivity, anti-interference ability and recyclability.


 Please wait while we load your content...
Please wait while we load your content...