High-spin enforcement in first-row metal complexes of a constrained polyaromatic ligand: synthesis, structure, and properties†
Abstract
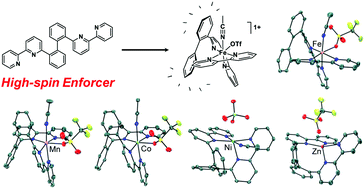
The coordination chemistry of a rigid tetradentate polypyridyl ligand has been developed with first-row transition metals Mn(II), Fe(II), Co(II), Ni(II), and Zn(II). The polyaromatic ligand, 2,2′-di([2,2′-bipyridin]-6-yl)-1,1′-biphenyl (5, bpbb), is comprised of 2,2′-bipyridine donors positioned at the 2 and 2′ carbons of a biphenyl backbone. Notably, coordination of the typical strong field bipyridine fragments is constrained, weakening the octahedral ligand fields around manganese, iron, and cobalt to give high-spin electronic states. Solution magnetic susceptibility measurements were conducted across the series using the Evans method and variable-temperature solid-state SQUID magnetometry was performed on two Fe(II) compounds, including a bis(thiocyanato) species. Spin crossover behavior was not observed as the compounds remained high-spin over the entire temperature range. The impact of the biphenyl bridge on M–N bond distances and redox potentials has also been assessed by comparison to relevant first-row metal bis- and tris-bipyridine compounds from the literature.


 Please wait while we load your content...
Please wait while we load your content...