Free-radical reaction synthesis of carbon using nitrogenous organic molecules and CCl4†
Abstract
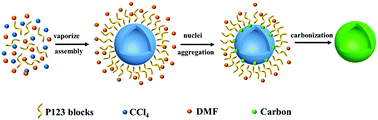
Carbonaceous materials are widely used materials in energy storage, biomedicine and electronics. Although many improvements had been achieved, the synthesis of carbon without using any metal catalyst or reducing agent is still a challenge, especially under low temperature conditions. In this paper, we illuminate a free radical mechanism for the synthesis of amorphous carbon hollow spheres in a large scale through the reaction between CCl4 and N,N-dimethylformamide (DMF) at 200 °C. The investigation of the reaction mechanism reveals the reaction possibility of CCl4 with other nitrogenous organic molecules, including dimethylacetamide (DMAC), hexamethylenetetramine (HMTA), diethylenetriamine (DETA), diethylamine (DEA), ethylenediamine (EN), and 1-methyl-2-pyrrolidinone (NMP). The present synthetic methodology provides a non-metal ion participating route to synthesize carbonaceous materials with organic molecules at low temperature.


 Please wait while we load your content...
Please wait while we load your content...