2-Amino-6-chloropurine-modified polyamidoamine-mediated p53 gene transfection to achieve anti-tumor efficacy†
Abstract
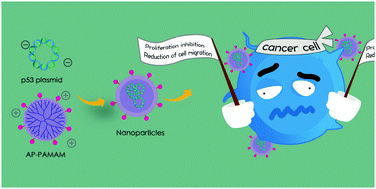
The modification of 2-amino-6-chloropurine on polyamidoamine was performed to synthesize a derivative, AP-PAMAM, which was then employed as a carrier for p53 gene delivery to achieve anti-tumor efficacy. Using a human prostate tumor cell line PC-3 as a model, enhanced expression level of p53 gene could be detected by RT-PCR, qPCR and western blotting. Based on the successful p53 gene transfection, an obvious anti-proliferative effect could be obtained, which was identified to be associated with the simultaneous induction of cell apoptosis and cell cycle arrest at the G1 phase. The cell apoptosis was elucidated to be triggered by the activation of the mitochondria-dependent apoptotic signaling pathway. In addition, AP-PAMAM/p53 transfection could restrain the migration of tumor cells as demonstrated by wound healing and Transwell migration assays. Notably, AP-PAMAM-mediated p53 delivery exhibited a stronger inhibitory effect on both cell proliferation and migration than the PAMAM/p53 group. In summary, AP-PAMAM/p53 transfection provided a promising route to achieve the p53-based gene therapy.


 Please wait while we load your content...
Please wait while we load your content...