Chiral separation and a molecular modeling study of eight azole antifungals on the cellulose tris(3,5-dichlorophenylcarbamate) chiral stationary phase
Abstract
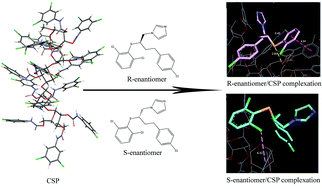
Four immobilized polysaccharide-based chiral stationary phases have been examined for their enantioselectivity on azole analytes using normal phase liquid chromatography. The resolving ability of the individual CSP differed in different mobile phase compositions and separated analytes. This article focuses on the comparison of the enantioselectivity on these columns. The effects of the side chain as well as the polymer backbone of the CSP on chiral discrimination are discussed. To gain a better understanding of the chiral recognition mechanism, detailed computational simulations of Chiralpak IC interacting with eight pairs of enantiomers were carried out using the molecular docking software AutoDock. The docking studies provided information about the binding energies and the conformations of chiral stationary phase–solute complexes. The chirality of an analyte molecule determines the number and strength of the intermolecular interactions which led to the differences in binding energies between the complexes formed by (R)- and (S)-isomers. These differences were in agreement with the observed enantioselectivity in HPLC experiments. It was found that the enantioselective separation of azole analytes on Chiralpak IC was the result of a mixture of hydrogen bond, π–π, hydrophobic and some special interactions while chromatographic retention was mainly affected by the polarity of the mobile phase. Furthermore, the chiral separation was also correlated to the molecular features, as the enantioselectivity of a more rigid molecule was relatively poor. Analytes having hydrophobic chlorine atoms, phenyl groups and hydrogen bond acceptors close to the chiral center were expected to be well resolved.


 Please wait while we load your content...
Please wait while we load your content...