New model of metalloantibiotic: synthesis, structure and biological activity of a zinc(ii) mononuclear complex carrying two enrofloxacin and sulfadiazine antibiotics†
Abstract
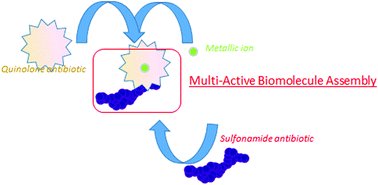
A new model of the Zn-based complex, [Zn(LH)(Erx)(ErxH)]ClO4 (sulfadiazine: LH and enrofloxacin ErxH), has been synthesised with two different, but complementary antibiotics (sulfonamide and quinolon) following an easy procedure. To evaluate the synergetic effect of this multicomponent molecule, the mononuclear complexes [Zn(L)2(H2O)(NH3)] and [Zn(Erx)3]− which each include only one of the two antibiotics (sulfadiazine: LH or enrofloxacin ErxH) were synthesized. All three compounds were characterized, including crystal structure determination by single-crystal X-ray diffraction. For all of the complexes, enrofloxacin is coordinated to Zn(II) by pyridinone and one oxygen atom of the carboxylate group in a monodente mode. The antibacterial activity, determined by the minimum inhibitory concentration on specific bacteria, has been studied on free ligands, on the corresponding mononuclear complexes and on our model.


 Please wait while we load your content...
Please wait while we load your content...