Peptide-based development of PKA activators†
Abstract
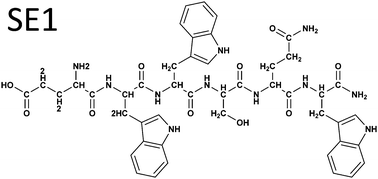
Protein kinases are among the most important regulators of cellular homeostasis, regulating many aspects of the cell life cycle through phosphorylation. The threonine/serine kinase cAMP-dependent protein kinase (PKA) is a major intracellular effector for a variety of bioactive molecules and hormones that work via a cAMP signaling pathway. In this work we report the discovery of a peptide and its simple peptidomimetic derivatives, which can activate the PKA catalytic unit. The direct binding of these new compounds to PKA and their resulting and activation mode were demonstrated by cellular (cardiomyocytes) and in vitro methods. Activation of PKA in cardiomyocytes after myocardial infarction might be a promising therapeutic strategy to protect the heart from ischemia and reperfusion. In addition, small-molecule PKA activators may prove useful as pharmacological tools for studying transduction mechanisms related to PKA. Several small molecules, which can activate a regulatory PKA unit, were developed. However, no small molecules that can activate a PKA catalytic unit were described in the scientific literature.


 Please wait while we load your content...
Please wait while we load your content...