A pyrazoline-based fluorescent chemosensor for Al3+ ion detection and live cell imaging†
Abstract
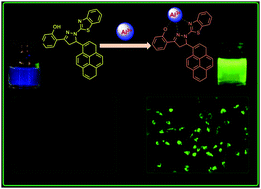
Two pyrene-appended pyrazoline compounds (PY-I and PY-II) were designed and synthesized. Their photophysical properties were studied by absorption and fluorescence spectral studies. PY-I displays an excellent selective and sensitive response to Al3+ ions over other metal ions, whereas PY-II did not respond to any metal ions. A 1 : 1 stoichiometry for the PY-I–Al3+ complex was observed with an association constant of 2.13 × 103 M−1. Furthermore, PY-I was employed for fluorescence imaging of Al3+ ions in living cells. PY-I has a phenolic group as a binding site at the 3-position of the pyrazoline ring which is responsible for its sensing of metal ions. Nevertheless, PY-II has no hydroxyl groups in the phenyl ring and was unable to sense metal ions; however, PY-II has good photophysical properties which make it a suitable fluorescent material.


 Please wait while we load your content...
Please wait while we load your content...