Exploration of fluorescent organotin compounds of α-amino acid Schiff bases for the detection of organophosphorous chemical warfare agents: quantification of diethylchlorophosphate†
Abstract
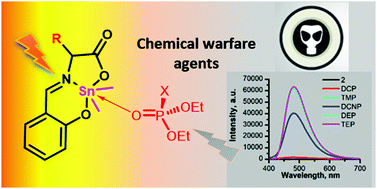
Herein, fluorescent di-methyltin(IV) compounds (1–4) of an azomethinic ligating system derived from the template assisted condensation of α-amino acids (i.e.L-alanine, L-leucine, L-glutamine, and L-isoleucine) and 2-hydroxybenzaldehyde are reported. The synthesized di-methyltin derivatives were characterized using FT-IR spectroscopy, multi-nuclei NMR (1H, 13C and 119Sn) spectroscopy, mass spectrometry and single crystal X-ray diffraction. The compounds 1–4 exhibited fluorescence emission centred at 470 nm when excited at a wavelength of 380 nm. The emission band experienced quenching in the presence of various organophosphates, therefore, a representative compound was explored for the fluorogenic chemo-sensing of various organophosphates. The fluorescence response was found to be maximum for diethylchlorophosphate (a potential mimic of chemical warfare agents) and offered detection up to 0.023 mM. To explore the practicality of the test compound, it was successfully incorporated into portable devices of variable designs, such as fluorescent strips and silica tablets, for the on-the-spot detection of chemical warfare agents.


 Please wait while we load your content...
Please wait while we load your content...