Synthesis of a deoxyguanosine monophosphate rich propyl methacrylate oligomer†
Abstract
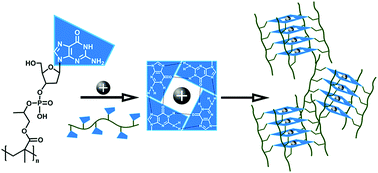
We report on the first synthesis of a “protected” 5′-dimethoxytrityl-N-isobutyryl-2′-deoxyguanosine(dG), 3′-[(2-cyanoethyl)-2-(methacryloyloxy)propyl]-monophosphate monomer through a modified phosphoramidite coupling method. Here, 2-hydroxylpropyl methacrylate (HPMA) was coupled to a protected 5′-dimethoxytrityl-N-isobutyryl-2′-deoxyguanosine, 3′-[(2-cyanoethyl)-(N,N-diisopropyl)]-phosphoramidite. Subsequent reversible addition fragmentation chain transfer (RAFT) polymerisation of the protected monomer, followed by deprotection, produced poly[2-(deoxyguanosine-3′-phospho)propyl methacrylate] (poly(dG-P-PMA)). In situ1H nuclear magnetic resonance spectroscopy showed the polymerisation kinetics were consistent with RAFT with dispersity indices (Đ's) <1.2. Circular dichroism showed that the isolated deprotected deoxyguanosine rich oligomers formed stacked G-quartets in both Li+ and K+ ion solutions. These structures were further investigated with confocal fluorescence microscopy.


 Please wait while we load your content...
Please wait while we load your content...
