New molecular heptanuclear cobalt phosphonates: synthesis, structures and magnetic properties†
Abstract
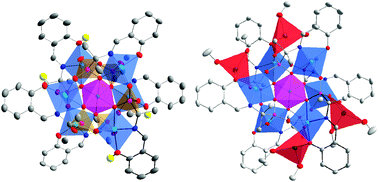
The synthesis, structures and magnetic properties of two novel heptanuclear homoleptic molecular cobalt phosphonates are described. Reactions between CoCl2·6H2O and (2-{[(E)-(2-hydroxyphenyl)methylidene]amino}propan-2-yl)phosphonate ligand (HSAA2−) in methanol lead to the crystalline products [Co7(SAA)2(HSAA)4] (1) and [Co7(SAA)2(NaSAA)4] (2), which crystallize in the space groups P21/n (1) and P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (2). Complexes show a similar structure motif – a centered trigonal antiprism composed of seven Co2+ ions with different coordination environments for the central and peripheral atoms. These heptanuclear molecular cages {Co7} are held together by six phosphonate ligands. The main difference in their molecular cores is the presence of four sodium cations in 2 instead of four phenolic protons in 1. Magnetic measurements reveal a strong magnetic anisotropy for both structures 1 and 2. Above 50 K, both complexes follow the Curie–Weiss law. The low temperature susceptibility data indicate differences in the intramolecular exchange coupling, as ferromagnetic and antiferromagnetic interactions are observed for 1 and 2, respectively. This can be related to structural differences leading to variations in the Co–O–Co bridging angles between the central octahedral and the six peripheral trigonal-bipyramidal Co2+ ions.
(2). Complexes show a similar structure motif – a centered trigonal antiprism composed of seven Co2+ ions with different coordination environments for the central and peripheral atoms. These heptanuclear molecular cages {Co7} are held together by six phosphonate ligands. The main difference in their molecular cores is the presence of four sodium cations in 2 instead of four phenolic protons in 1. Magnetic measurements reveal a strong magnetic anisotropy for both structures 1 and 2. Above 50 K, both complexes follow the Curie–Weiss law. The low temperature susceptibility data indicate differences in the intramolecular exchange coupling, as ferromagnetic and antiferromagnetic interactions are observed for 1 and 2, respectively. This can be related to structural differences leading to variations in the Co–O–Co bridging angles between the central octahedral and the six peripheral trigonal-bipyramidal Co2+ ions.


 Please wait while we load your content...
Please wait while we load your content...