Crystal structure, physicochemical properties, Hirshfeld surface analysis and antibacterial activity assays of transition metal complexes of 6-methoxyquinoline†
Abstract
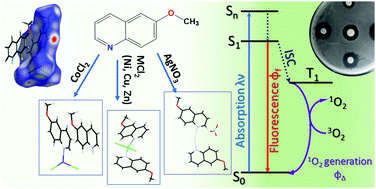
Five monomeric complexes of Co(II), Cu(II), Ni(II), Zn(II) and Ag(I) with 6-methoxyquinoline (6-MeOQ) as ligand have been prepared, and their crystal structures have been determined by single X-ray diffractions. The Cu(II), Ni(II) and Zn(II) complexes are formulated as M(6-MeOQ)2Cl2, completing MN2Cl2 coordination spheres. On the other hand, Co(II) and Ag(I) compounds are ionic with formulae [Ag(6-MeOQ)2]+ NO3− and H(6-MeOQ)+[Co(6-MeOQ)Cl3]− (where H(6-MeOQ)+ is the protonated ligand). Hirshfeld surface analysis was employed to study the intermolecular interactions in the crystal lattices and from these studies it was found that π-stacking contacts play an important role. Besides, the complexes have been characterized by FTIR, UV-visible and emission spectroscopies. The singlet oxygen production and fluorescence quantum yields were measured for all the complexes employing steady-state methodologies. Finally, the antibacterial activity of the complexes was screened against both Gram-positive and Gram-negative bacteria.


 Please wait while we load your content...
Please wait while we load your content...