trans-A2B-corrole bearing 2,3-di(2-pyridyl)quinoxaline (DPQ)/phenothiazine moieties: synthesis, characterization, electrochemistry and photophysics†
Abstract
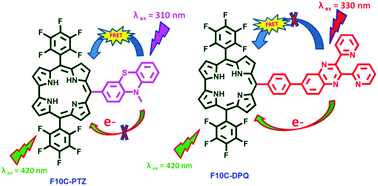
Two novel donor–acceptor systems (F10C–PTZ and F10C–DPQ) were designed and synthesized, in which corrole is an acceptor and either phenothiazine (PTZ) or 2,3-di(2-pyridyl)quinoxaline (DPQ) is an energy/electron donor. These dyads represent some of the few examples of photostable corrole dyads reported in the literature, but the first example of 2,3-di(2-pyridyl)quinoxaline (DPQ) containing meso substituted corrole (F10C–DPQ) also directly connected PTZ to the meso position of corrole (F10C–PTZ). Both the dyads were characterized by high-resolution mass spectrometry (HR-MS), NMR (1H, 13C, 19F) spectroscopy, UV-Vis spectroscopy, steady state fluorescence, time-resolved fluorescence (TCSPC, MCP-PMT), electrochemical methods (CV, DPV) as well as theoretical calculations (DFT, TD-DFT). From absorption and electrochemical studies it is evident that there exist minimum π–π interactions between two hetero chromophoric units in the dyads. The steady state fluorescence studies indicate that an efficient quenching of emission corresponding to the PTZ unit in the F10C–PTZ dyad is attributed to the singlet–singlet energy transfer from PTZ to corrole. In addition to the spectral overlap, excitation spectra provided additional evidence for intramolecular energy transfer. Whereas in the F10C–DPQ dyad, a reductive electron transfer, from the ground state of DPQ to the excited state of corrole, was observed. The negative free energy (ΔG) for charge separation, anodic shift of reduction potential of corrole and the frontier molecular orbital (LUMO) location provide addition support for charge transfer. The solvent dependent rate of an energy and electron transfer was discussed.


 Please wait while we load your content...
Please wait while we load your content...