A theoretical study of new representatives of closed- and open-circle benzofuran and benzocyclopentadienone oligomers†
Abstract
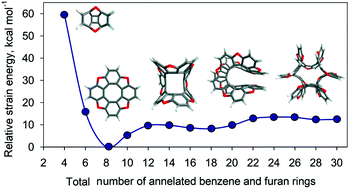
Owing to their potential use as materials for strong optical rotation, circular dichroism and circularly polarized luminescence, the structure and energetic stability of novel closed- and open-circle benzofuran and benzocyclopentadienone oligomers have been studied computationally using the DFT method. It is found that an extension of the macrocycle size (n = 10–30) for the closed-circle species leads to a gradual transformation of their structure from saddle- to helical- and then to a wing-like shape. At the same time, the smaller closed-circle representatives with n = 4 and 6 exhibit a bowl-shaped structure due to the high energy strain of their molecular structures. Application of the NICS (nucleus-independent chemical shift) criterion to the here studied open-circle benzofuran and benzocyclopentadienone species with planar linear, planar fan-shape and helical molecular topology indicates that for the planar benzofuran series the inner benzene ring is more aromatic than the outer hexagons, while the aromaticity of the outer and inner furan rings is insensitive to the molecular size variations. The replacement of the aromatic furan rings by antiaromatic cyclopentadienone fragments for the planar linear species leads to a decrease of aromaticity of the benzene rings from the edge to the center of the benzocyclopentadienone chain. On the other hand, for the planar fan-shape benzocyclopentadienone series a zigzag trend in the NICS values is observed for the benzene rings. Loss of planarity only slightly affects the aromaticity of the helical open-circle benzofuran and benzocyclopentadienone oligomers. We propose that an increase of the NICS indices for the outer and inner rings of the macromolecular helical species is due to the magnetic couplings between the superimposed benzene and furan/cyclopentadienone rings.


 Please wait while we load your content...
Please wait while we load your content...