Competition between hydrogen bonds and halogen bonds: a structural study†
Abstract
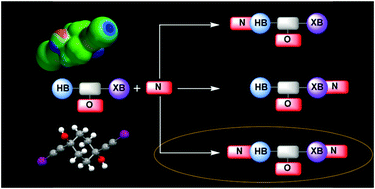
The competition and balance between intermolecular hydrogen bonds (HBs) and halogen bonds (XBs) were explored by co-crystallizing tetra-functionalized (2 × HB (–OH) and 2 × XB (–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–I)) molecules, trans-1,4-bis(iodoethynyl)cyclohexane-1,4-diol (D1) and cis-1,4-bis(iodoethynyl)cyclohexane-1,4-diol (D2), with six ditopic nitrogen based acceptor molecules. The crystal structures of both D1 and D2 showed non-covalent interactions between HB/XB donors and available acceptor sites (oxygen/triple bond/negative region of iodine). In three co-crystals of D1 the HB and XB donors act in similar ways as both activated iodine and hydroxyl hydrogen bind to the nitrogen acceptors in the solid state. In contrast, in a co-crystal of D2, a geometric isomer of D1, there were only hydrogen bonds to the co-former and the halogen-bond donor interacted with the hydroxyl oxygen atoms of D2. A stronger tendency for linear XB interactions (as well as greater van der Waals radii reduction) was observed with nitrogen atoms as acceptors (average reduction = 21%) compared to those involving an oxygen atom as an acceptor (average reduction = 16%). A control molecule, trans-1,4-diethynylcyclohexane-1,4-diol (D3), which has only HB donors (–OH and –C
C–I)) molecules, trans-1,4-bis(iodoethynyl)cyclohexane-1,4-diol (D1) and cis-1,4-bis(iodoethynyl)cyclohexane-1,4-diol (D2), with six ditopic nitrogen based acceptor molecules. The crystal structures of both D1 and D2 showed non-covalent interactions between HB/XB donors and available acceptor sites (oxygen/triple bond/negative region of iodine). In three co-crystals of D1 the HB and XB donors act in similar ways as both activated iodine and hydroxyl hydrogen bind to the nitrogen acceptors in the solid state. In contrast, in a co-crystal of D2, a geometric isomer of D1, there were only hydrogen bonds to the co-former and the halogen-bond donor interacted with the hydroxyl oxygen atoms of D2. A stronger tendency for linear XB interactions (as well as greater van der Waals radii reduction) was observed with nitrogen atoms as acceptors (average reduction = 21%) compared to those involving an oxygen atom as an acceptor (average reduction = 16%). A control molecule, trans-1,4-diethynylcyclohexane-1,4-diol (D3), which has only HB donors (–OH and –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–H) was also examined to get a better understanding of the balance between XB and HB intercations. The ethynyl hydrogen atom did not form hydrogen bonds to the nitrogen atoms in acceptors, and only O–H⋯N and –C
C–H) was also examined to get a better understanding of the balance between XB and HB intercations. The ethynyl hydrogen atom did not form hydrogen bonds to the nitrogen atoms in acceptors, and only O–H⋯N and –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–H⋯O hydrogen bonds were observed in these structures.
C–H⋯O hydrogen bonds were observed in these structures.
- This article is part of the themed collection: The halogen bond: a new avenue in recognition and self-assembly


 Please wait while we load your content...
Please wait while we load your content...