Extended lead(ii) architectures engineered via tetrel bonding interactions†
Abstract
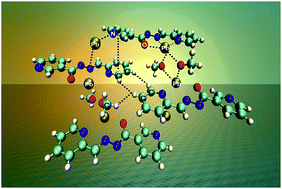
The evaluation of N′-(pyridin-2-ylmethylene)nicotinohydrazide (HLI) and N,N′′′-bis(1-(pyridin-2-yl)-ethylidene)carbazide (H2LII) as linker precursors in the synthesis of novel PbII extended structures is described. An equimolar one-pot reaction of PbX2 (X = NO3−, CH3COO−) salts with HLI and H2LII in MeOH at 60 °C in a branched tube apparatus leads to heteroleptic complexes [Pb(HLI)(NO3)2]n (1), [Pb(LI)(CH3O)]n (2), [Pb2(H2LII)(NO3)4] (3) and [Pb2(HLII)(CH3COO)3]n (4), respectively. The nature of the anion in the parent PbII salt also influences the final structure. In all complexes, the PbII center exhibits a hemidirected coordination geometry with all the covalent bonds being concentrated on one hemisphere of the coordination sphere. The sterically available PbII ion participates in Pb⋯O/N tetrel bonding or Pb⋯Cg interaction as evidenced from the detailed structural and topological analysis of the described complexes. As a result of these interactions, the structures of all four compounds can be extended to a higher dimensional framework, which is further stabilized by hydrogen N–H⋯O/C–H⋯O and dihydrogen C–H⋯H–C bonds and/or π⋯π stacking interactions. The complementary Hirshfeld surface analysis of the discrete complex 3, considering covalent bonds, showed that the structure is highly dominated by H⋯X (X = O, H and C) and O⋯Y (Y = O, Pb, C and N) contacts, of which the O⋯Pb/H/N/C contacts are highly favoured. DFT based charge and energy decomposition (ETS-NOCV) calculations are performed in order to shed light on the nature of the non-covalent interactions that determine the stability of the obtained structures.


 Please wait while we load your content...
Please wait while we load your content...