Extraction performance of Eu3+ by using heterocyclic N-donor ligands with different structures in ionic liquids: an experimental and theoretical study†
Abstract
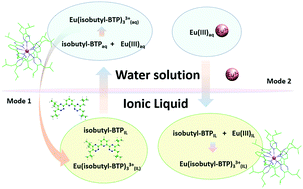
The extraction performance of 2,6-bis(5,6-diisobutyl-1,2,4-triazin-3-yl)pyridine (isobutyl-BTP), 2,6-bis(5,9,9-trimethyl-5,6,7,8-tetrahydro-5,8-methanobenzo[1,2,4]triazin-3-yl)pyridine (CA-BTP) and 2,9-bis(5,9,9-trimethyl-5,6,7,8-tetrahydro-5,8-methanobenzo[1,2,4]triazin-3-yl)-1,10-phenanthroline (CA-BTPhen) in combination with 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([C2mim][NTf2]) in the presence of nitric acid was investigated by using Eu3+ as the model ion, which has similar properties to trivalent actinides. It was found that both nitric acid and the structure of heterocyclic N-donor ligands have influence on the extraction performance of Eu3+. With the increment of nitric acid concentration, the dominant extraction mechanism varies from the cation exchange mechanism to the neutral complex mechanism. Isobutyl-BTP has the fastest extraction rate, and CA-BTPhen has the highest extraction ratio both under neutral conditions and at 0.01 M nitric acid. DFT calculations demonstrated that the protonation and conformational change of heterocyclic N-donor ligands are related to their structure, which further influence the structure and migration of extracted species during extraction. During the extraction process, heterocyclic N-donor ligands prefer to migrate to the aqueous phase and form a complex with Eu3+, and then the coordination complexes migrate back to the ionic liquid phase.


 Please wait while we load your content...
Please wait while we load your content...