Facile synthesis of functionalized urea, imidazolium salt, azide, and triazole from a 2-amino-5,7-dimethyl-1,8-naphthyridine scaffold and their utilization in fluoride ion sensing†
Abstract
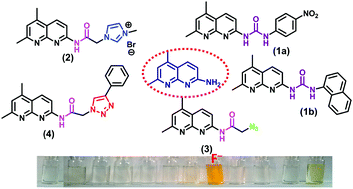
Four new 2-amino-5,7-dimethyl-1,8-naphthyridine derivatives (1–4) possessing urea, amide-imidazolium salt, amide-azide, or amide-triazole moieties were synthesized in good to excellent yields by derivatization of 2-amino-5,7-dimethyl-1,8-naphthyridine. We examined their anion recognition abilities towards different anions such as fluoride, chloride, bromide, iodide, nitrate, dihydrogen phosphate, cyanide, hexafluorophosphate, perchlorate, hydrogen sulphate and acetate by 1H NMR and UV-Vis spectroscopy. Among various 2-amino-5,7-dimethyl-1,8-naphthyridine derivatives, only 1a and 2 showed spectroscopic and colorimetric change when treated with fluoride ions among other anions. The F− ions first established a hydrogen-bonding interaction with 1a to give the most stable 1 : 1 complex and then, after addition of a second equivalent, the F− ions induced urea deprotonation due to the formation of HF2−. Moreover, 2 underwent deprotonation of amide –NH proton after the addition of 1 equiv. of fluoride ions. The action of the probes was thoroughly investigated by DFT calculations that also supported the H-bonding induced deprotonation sensing mechanism.


 Please wait while we load your content...
Please wait while we load your content...