Cyanine dyes: synergistic action of hydrogen, halogen and chalcogen bonds allows discrete I42− anions in crystals†
Abstract
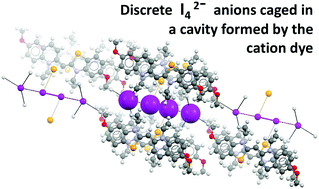
In crystals of a benzoselenazole dye, a network of hydrogen bonds with the dye cations locks two iodide anions at a distance allowing for an iodine molecule to be pinned at either ends via two halogen bonds. I42− supramolecular anions are formed as discrete species that lie in the confined space defined by cation molecules.
- This article is part of the themed collection: The halogen bond: a new avenue in recognition and self-assembly


 Please wait while we load your content...
Please wait while we load your content...