Study of the structure–bioactivity relationship of three new pyridine Schiff bases: synthesis, spectral characterization, DFT calculations and biological assays†
Abstract
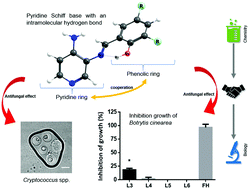
Schiff bases exhibit a broad range of applications, including their use as catalysts, stabilizers, dyes, and intermediates in organic synthesis; and biological activities, such as antifungal properties. In this work, we synthesized and characterized three new pyridine Schiff bases L3 ((E)-2-{[(3-aminopyridin-4-yl)imino]-methyl}-4,6-di-chloro-phenol), L4 ((E)-2-{[(3-aminopyridin-4-yl)imino]-methyl}-6-chloro-phenol), and L5 ((E)-2-{[(3-aminopyridin-4-yl)imino]-methyl}-4-methyl-phenol) to explore their structure–bioactivity relationship as antifungal agents. We also synthesized and characterized a diamine-derived Schiff base (L6) that is similar to (E)-2-{[(3-aminopyridin-4-yl)imino]-methyl}-4,6-di-tert-butyl-phenol (L2, which previously demonstrated antifungal activity), but lacks the pyridine ring, to assess the impact of this structural modification on the biological properties. All the Schiff bases were characterized by FTIR, 1H and 13C NMR, DEPT, HHCOSY, TOCSY, UV-vis, MS, cyclic voltammetry, DFT calculations, and NBO to assess the stability of the intramolecular hydrogen bond (IHB). In addition, we determined the antimicrobial properties by obtaining the minimal inhibitory concentration (MIC) for Cryptococcus spp. (yeast) and Salmonella enterica (bacteria), and growth inhibition of Botrytis cinerea (mold). We found that the antifungal activity of these Schiff bases relied on the nitrogen atom in the pyridine ring, and the antifungal activity can be modulated by different substituents in the phenolic ring. In this work we provide data supporting a correlation between the structure and bioactivity in this kind of Schiff base. The understanding of the structural prerequisites for antimicrobial activity could contribute to designing new drugs.


 Please wait while we load your content...
Please wait while we load your content...