Peptides N-connected to hydroxycoumarin and cinnamic acid derivatives: synthesis and fluorescence spectroscopic, antioxidant and antimicrobial properties†
Abstract
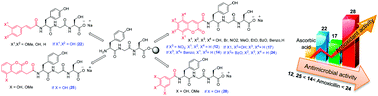
The tripeptide Tyr–Gly–Ser and a series of conjugations to coumarin, cinnamic and gallic acid were synthesized in salt form and their antioxidant and antimicrobial activities were investigated. The N-connecting hydroxycoumarin, cinnamic and gallic acid derivatives to peptides and the use of BBr3 as a demethylating agent for peptides was reported. Their activities were investigated based on the conjugated moiety structures. Studies of their activities showed that conjugated tripeptides 7,8-dihydroxycoumarin-peptide (17), caffeic acid-peptide (22) and gallic acid-peptide (28) were found to be superior to ascorbic acid with respect to their antioxidant activity, and 12, 14, 24, and 25 exhibited the most antimicrobial activity in the series compared to amoxicillin. Additionally, the incredible florescence intensity and brightness of 17 in water and DMSO, compared to other synthesized compounds, qualified this peptide as a suitable probe in the human body.


 Please wait while we load your content...
Please wait while we load your content...