A photo-stable fluorescent chiral thiourea probe for enantioselective discrimination of chiral guests†
Abstract
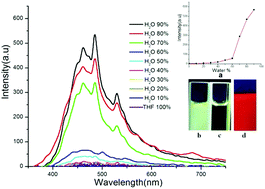
Herein, a chiral thiourea Schiff base derived from (1R,2R)-1,2-cyclohexanediamine and tetraphenylethylene (TPE) was applied as a highly effective chiral sensor for the enantioselective discrimination of various acids and amines via ion-pair and hydrogen-bond interaction. Compared to the case of the sensors 5 and 6, the additional thiourea and hydrogen groups of sensor 4 were essential and greatly enhanced the enantioselectivity of chiral guests. In addition, for amino acids, the sensor 4 showed high enantioselectivity, and precipitates visible by the naked eye appeared; however, for chiral amines, the enantioselectivity decreased. This was attributed to weaker hydrogen bond interaction between the amino groups of chiral amines and the sensor. Both the thiourea and acidic hydroxyl groups are essential for chiral TPE thiourea to provide an appropriate chiral environment for highly efficient enantioselective discrimination of chiral substrates. Our findings will be of great value in the design of new chiral sensors.


 Please wait while we load your content...
Please wait while we load your content...