A conjugated BODIPY–triphenylamine multi-aldoxime: Sonogashira coupling, ratiometric chemodosimeter and rapid detection of hypochlorite with two-photon excited fluorescence†
Abstract
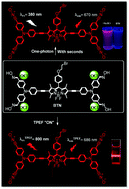
A conjugated BODIPY–triphenylamine multi-aldoxime (BTN) was designed and synthesized by the Sonogashira coupling reaction. The chemodosimeter showed a red emission (670 nm), fast response (within seconds), high selectivity and good sensitivity towards hypochlorite based on the redox reaction of four aldoximes. BTN possessed two original absorption peaks at 366 nm and 588 nm, respectively, corresponding to fluorescence emissions at 440 nm and 660 nm, which exhibited a ratiometric change in the presence of hypochlorite. With the addition of hypochlorite, the redox reaction of aldoxime led to the termination of isomerization of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond, the fluorescence intensity at 660 nm gradually changed from weak to strong, accompanied by a slight 10 nm red-shift; in contrast, the fluorescence intensity began to decrease at 440 nm. Moreover, as the two-photon excited fluorescent NaOCl probe, BTN afforded an enhanced signal response for hypochlorite at 686 nm under two-photon excitation (λTPEFex = 800 nm). Meanwhile, successful imaging of living cells was carried out for BTN towards hypochlorite in A-549 cells. The results demonstrated that BTN had potential application prospects and was promising for practical application in biological systems and environmental systems.
N bond, the fluorescence intensity at 660 nm gradually changed from weak to strong, accompanied by a slight 10 nm red-shift; in contrast, the fluorescence intensity began to decrease at 440 nm. Moreover, as the two-photon excited fluorescent NaOCl probe, BTN afforded an enhanced signal response for hypochlorite at 686 nm under two-photon excitation (λTPEFex = 800 nm). Meanwhile, successful imaging of living cells was carried out for BTN towards hypochlorite in A-549 cells. The results demonstrated that BTN had potential application prospects and was promising for practical application in biological systems and environmental systems.


 Please wait while we load your content...
Please wait while we load your content...