Three new super water-stable lanthanide–organic frameworks for luminescence sensing and magnetic properties†
Abstract
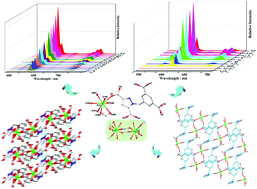
Three new lanthanide compounds, [Ln(HL)(H2O)3]n (1-Ln) (Ln = Eu, Tb and Dy), with isostructural structures have been constructed by a semi-rigid ligand 1-(3,5-dicarboxylatobenzyl)-3,5-pyrazole dicarboxylic acid (H4L) and Ln(NO3)3·6H2O using the solvothermal method. Three compounds show two-dimensional (2D) grid layer-stacking frameworks with interlayer channels decorated with carboxylic acid groups. 1-Eu exhibits excellent water stability and strong luminescence. Importantly, as a probe for sensing different metal ions and anions, 1-Eu shows highly specific recognition to Fe3+, CrO42− and Cr2O72− ions. Meanwhile, the mechanisms of luminescence quenching were studied, and antiferromagnetic exchange interactions existed in 1-Dy.


 Please wait while we load your content...
Please wait while we load your content...