From 4-nitrotoluene and 4,4′-dinitrobibenzyl to E-4,4′-dinitrostilbene: an electrochemical approach
Abstract
The dianions formed by the electroreduction of Z-O2NC6H4CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC6H4NO2, E-O2NC6H4CH
CHC6H4NO2, E-O2NC6H4CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC6H4NO2, O2NC6H4CH2–CH2C6H4NO2 or O2NC6H4CMeH–CMeHC6H4NO2, as well as the anion radical arising from 4-nitrotoluene, are stable, in the time scale of cyclic voltammetry (DMF + 0.10 M NBu4BF4). However, in the electrolysis time scale (from minutes to hours), only the dianion O2NC6H4CMeH–CMeHC6H4NO22− remains stable, since the reduced species, Z-O2NC6H4CH
CHC6H4NO2, O2NC6H4CH2–CH2C6H4NO2 or O2NC6H4CMeH–CMeHC6H4NO2, as well as the anion radical arising from 4-nitrotoluene, are stable, in the time scale of cyclic voltammetry (DMF + 0.10 M NBu4BF4). However, in the electrolysis time scale (from minutes to hours), only the dianion O2NC6H4CMeH–CMeHC6H4NO22− remains stable, since the reduced species, Z-O2NC6H4CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC6H4NO22−, O2NC6H4CH2–CH2C6H4NO22− or O2NC6H4Me˙−, evolve to form the E-O2NC6H4CH
CHC6H4NO22−, O2NC6H4CH2–CH2C6H4NO22− or O2NC6H4Me˙−, evolve to form the E-O2NC6H4CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC6H4NO22− dianion. This intermediate is recovered as the neutral species E-O2NC6H4CH
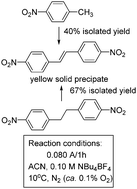
CHC6H4NO22− dianion. This intermediate is recovered as the neutral species E-O2NC6H4CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC6H4NO2 with concomitant water reduction after the work-up with water, as demonstrated by combined electrolysis, cyclic voltammetry experiments, UV-spectroelectrochochemistry and theoretical calculations. Bulk electrolysis under optimized conditions (ACN + 0.10 M NBu4BF4) provides 40% and 67% isolated yields of E-4,4′-dinitrostilbene from 4-nitrotoluene and 4,4′-dinitrobibenzyl, respectively.
CHC6H4NO2 with concomitant water reduction after the work-up with water, as demonstrated by combined electrolysis, cyclic voltammetry experiments, UV-spectroelectrochochemistry and theoretical calculations. Bulk electrolysis under optimized conditions (ACN + 0.10 M NBu4BF4) provides 40% and 67% isolated yields of E-4,4′-dinitrostilbene from 4-nitrotoluene and 4,4′-dinitrobibenzyl, respectively.


 Please wait while we load your content...
Please wait while we load your content...