MCR-click synthesis, molecular docking and cytotoxicity evaluation of a new series of indole–triazole–coumarin hybrid peptidomimetics†
Abstract
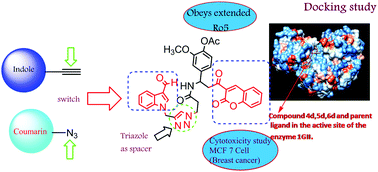
The design and synthesis of a new series of indole–triazole–coumarin hybrids as potential CDK2 inhibitors is described. The new hybrid molecules were synthesized via copper(I) catalyzed [3+2] azide–alkyne cycloaddition and showed excellent binding affinity towards CDK2 kinase when subjected to virtual screening based on molecular docking. The molecular docking results were experimentally validated by cytotoxicity evaluation against human breast cancer cell line MCF-7 and western blot analysis. The IC50 value (17.5 μM) and binding affinity (−11.2 kcal mol−1) obtained for 6a against MCF-7 cells are promising for the development of potential anticancer drugs based on these new molecules.


 Please wait while we load your content...
Please wait while we load your content...