Iron, iron everywhere: synthesis and characterization of iron 5,10,15-triferrocenylcorrole complexes
Abstract
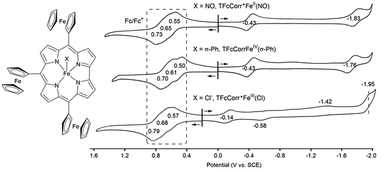
The chloroiron complex of 5,10,15-triferrocenylcorrole was synthesized by direct metalation of the crude free-base corrole product obtained by the reaction of ferrocenyl aldehyde with pyrrole. This procedure eliminates the need to isolate the unstable free-base corrole and gives a reasonable yield for the desired product, as earlier reported for structurally related Cu and Co derivatives. The isolated chloroiron corrole was then subjected to axial ligand exchange, leading to the generation of a diamagnetic iron nitrosyl complex and a paramagnetic σ-phenyl-bonded corrole derivative. The three related corrole complexes were then characterized using 1H NMR, electrochemical and spectroelectrochemical techniques.
- This article is part of the themed collection: Equilibrium Solution Coordination Chemistry


 Please wait while we load your content...
Please wait while we load your content...