A series of Ln2 complexes based on an 8-hydroxyquinoline derivative: slow magnetization relaxation and photo-luminescence properties†
Abstract
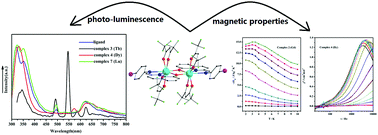
The reactions of 2-[(4-bromo-phenylimino)-methyl]-quinolin-8-ol (HL) and Ln(hfac)3·2H2O (Ln(III) = Pr, Gd, Tb, Dy, Ho, Er, Lu; hfac− = 1,1,1,5,5,5-hexafluoroacetylacetonate) precursors lead to the formation of seven lanthanide complexes with the formula of [Ln2(hfac)4L2] (Ln = Pr (1); Gd (2); Tb (3); Dy (4); Ho (5); Er (6); Lu (7)). The X-ray structural analysis shows that complexes 1–7 are phenoxo-bridged dinuclear complexes. The luminescence spectra in methanol solution show that complex 3 exhibits a characteristic Tb(III) centered luminescence, while complex 7 exhibits emission bands similar to that of the ligand. In addition, the magnetic properties of 2–6 are studied: the maximum value of −ΔSm for complex 2 is 15.00 J K−1 kg−1, while complex 4 shows a slow magnetic relaxation behaviour with an energy barrier of 4.25 K and a pre-exponential factor of 6.55 × 10−6 s.


 Please wait while we load your content...
Please wait while we load your content...