Redox-active diaminoazobenzene complexes of rhodium(iii): synthesis, structure and spectroscopic characterization†
Abstract
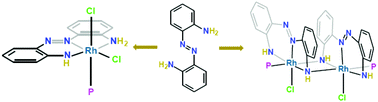
Herein, the reactivity of an aromatic diamine, 2,2′-diaminoazobenzene 1 [H2NLNH2], with rhodium salts was investigated. Diverse coordinations, specifically single and double NH deprotonated forms, of the title ligand are obtained during metallation, affording mononuclear 2 and dinuclear 3 complexes, respectively. An unprecedented edge-shared bioctahedral geometry with a syn configuration (sterically encumbered) about the RhIII2N2 core is obtained with pincer-type NNN ligation. Both complexes are redox-active and provide well-defined oxidative responses, and the reversibility of the redox-couples is enhanced with the decreasing temperature. The spectroscopic study indicates the formation of open-shell species derived from the precursor amido complexes, and these species are believed to be ligand-centered π radicals. The incorporation of both electron-poor azo and electron-rich amido moieties imparts the possibility of low-energy electronic transitions within the tridentate ligand (the push–pull effect). Furthermore, the occurrence of ILCT has been substantiated theoretically (TDDFT and NTO).


 Please wait while we load your content...
Please wait while we load your content...