Abstract
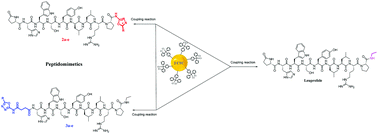
We were interested in the design and synthesis of novel bioisosteric analogues of leuprolide acetate containing the oxadiazole moiety at the C- or N-terminal of the peptide. An efficient approach for the synthesis of 2-amino-1,3,4-oxadiazoles through the reaction of hydrazide with ammonium thiocyanate and desulfurization reaction of the thiosemicarbazides using different coupling reagents was employed. These compounds are bioisosteres of the amide bond. Furthermore, the coupling of 2-amino-1,3,4-oxadiazoles at the C-terminal of leuprolide analogues was carried out, using a coupling reagent in the solution phase. On the other hand, the addition of a 2-amino-1,3,4-oxadiazole to the N-terminal of the peptide sequence was carried out through the reaction of the 2-amino-1,3,4-oxadiazole with succinic anhydride that led to the formation of a carboxylic acid moiety. Addition of the synthesized oxadiazole containing carboxylic acid to the peptide sequence was performed using a coupling reagent and on the surface of the resin. The synthesized peptides containing the oxadiazole moiety at the C- or N-terminal of the peptide sequence are peptidomimetics of leuprolide acetate. All of the synthesized peptides were purified using preparative HPLC and their structures were confirmed using HR-MS (ESI).


 Please wait while we load your content...
Please wait while we load your content...