Synthesis of thiomers: screening and optimization using chemometrics
Abstract
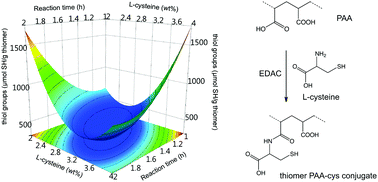
A chemometric study of the processing variables involved in the synthesis of thiomers based on poly(acrylic acid) functionalized with L-cysteine (PAA-cys) mediated by carbodiimide was successfully carried out. Immobilization of thiol groups in the polymer chain was confirmed using FTIR and TGA analysis. In order to obtain PAA-cys conjugates with a maximum immobilized thiol group content, a two-step approach using design of experiments was used. First, a two-level screening Plackett–Burman design was used to identify the most important processing variables: X1 the PAA molecular weight; X2 the concentration of PAA; X3 the concentration of carbodiimide; X4 the concentration of L-cysteine; X5 the activation time for carbodiimide; and X6 the reaction time. Secondly, a five-level D-optimal design was used in order to find the optimal levels of the most important variables to obtain the maximum thiol group content. Both screening and optimization models were ANOVA validated (P < 0.05) with a goodness of the model R2 > 0.980 and a goodness of prediction Q2 > 0.948. The optimized formulation presented 2442.2 μmol SH per g thiomer, reaching ∼215% more than the maximum amount reported in previous works obtained using a univariate approach. Also, the optimized PAA-cys conjugate achieved ∼5-fold more water uptake which is directly correlated with mucoadhesiveness.


 Please wait while we load your content...
Please wait while we load your content...