A hydrazine-based thiocarbamide probe for colorimetric and turn-on fluorometric detection of PO43− and AsO33− in semi-aqueous medium†
Abstract
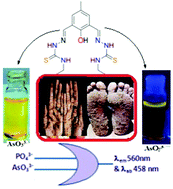
2,6-Bis(N-ethylhydrazonethiocarbamide)-4-methyl-phenol (HTP) recognizes PO43− and AsO33− and is effective as a colorimetric and turn-on fluoremetric sensor in the presence of a large number of biologically important cations and anions. The limit of detection (LOD) is 34 nM for PO43− and 15 nM for AsO33−. The composition of the probe–anion association has been established by Job's plot, 1H-NMR titration and HR-MS data. The Density Functional Theory (DFT) computation technique is used to optimize the structure that has been compared with an X-ray structure of HTP and the calculated electronic properties are correlated with the experimental results. Turn-on detection of PO43− and AsO33− is also successfully utilized for the fabrication of an ‘OR’ gate. The probe, HTP, is used for analysis of the ground water of Jadavpur, Kolkata.


 Please wait while we load your content...
Please wait while we load your content...