Diastereo- and enantioselective nitro-Mannich reaction of isatin-derived N-Boc ketimines catalyzed by chiral phase-transfer catalysts†
Abstract
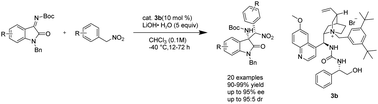
A highly diastereo- and enantioselective nitro-Mannich reaction of isatin-derived ketimines with α-aryl nitromethane catalyzed by Cinchona alkaloid-derived phase-transfer catalysts bearing multiple hydrogen-bonding donors (the first metal-free catalytic systems used in this reaction) was developed. A series of 3-substituted 3-amino-oxindoles were constructed using this protocol in excellent yields (96–99%) with high enantioselectivities (up to 95% ee) and diastereoselectivities (up to 95 : 5 dr).


 Please wait while we load your content...
Please wait while we load your content...