Highly efficient organocatalysts for the asymmetric aldol reaction†
Abstract
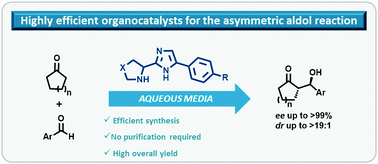
A new class of bifunctional organocatalysts containing both thiazolidine/pyrrolidine and imidazole cycles was prepared via a readily available synthetic route. The highly efficient five step methodology did not require intermediary purification and provided the compounds in high yields. The catalysts were successfully applied in the asymmetric direct aldol reaction between aromatic aldehydes and cyclic ketones in aqueous media. Aldol adducts were obtained in high yields with perfect stereoselectivities (up to >99% e.e. and >19 : 1 d.r.).


 Please wait while we load your content...
Please wait while we load your content...