Palladium-catalyzed three-component reaction for the synthesis of 3,3-disubstituted allylic alcohols with regio- and stereoselectivity†
Abstract
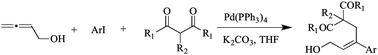
A novel three-component assembly of allenic alcohols, aryl iodides and 1,3-dicarbonyl compounds into 3,3-disubstituted allylic alcohols was promoted in the presence of a palladium source. A plausible mechanism through Pd0 catalysis was established to explain the regio- and stereoselectivity. Various aryl iodides and functionalized dicarbonyls were well-tolerated to afford products in good to moderate yields.


 Please wait while we load your content...
Please wait while we load your content...