Functional phenylethynylene side arm poly(arylene ethynylene) conjugated polymers: optical and electrochemical behavior for enrichment of electronic applications†
Abstract
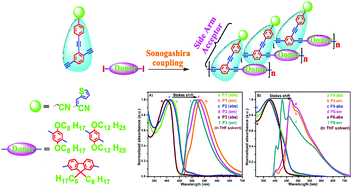
The poly(arylene ethynylene) (PAE) conjugated polymers (CPs) with a donor–acceptor (D–A) side arm have been designed and synthesized using Sonogashira cross coupling in the presence of cyano methylene, or cyano thiophene gave diethynyl (A) and alkoxy and alkyl substituted diiodo aryl monomers (D). An interesting electronic response in optical measurements such as UV-visible (UV-vis) and fluorescence (FL) spectra was observed in tetrahydrofuran solvent. From the FL spectra, it was observed that the CP solutions possess an interesting long bathochromic shift when compared with the UV-vis spectra, because of the electron withdrawing, electron releasing and conjugation effects. The electrochemical and thin film UV-vis spectral measurements provided highest occupied molecular orbital-lowest unoccupied molecular orbital (HOMO–LUMO) electronic energy levels and their corresponding semiconducting electronic energy gaps (Eg) of the PAE CPs. Among the side arm CPs, polymers P1 and P5 have low Eg of 2.14 eV and 2.17 eV. The new PAE CPs are reliable for use in electronic and photonics applications.


 Please wait while we load your content...
Please wait while we load your content...