Exploiting direct heteroarylation polymerization homocoupling defects for the synthesis of a molecular dimer
Abstract
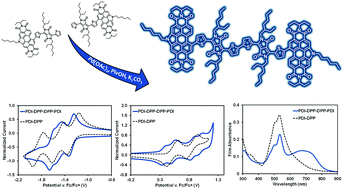
We have utilized a C–H homocoupling defect that plagues direct heteroarylation polymerization reactions to access a molecular dimer based on diketopyrrolopyrrole and perylene diimide. This synthetic route, which also incorporates a direct heteroarylation mono-substitution, proved to be an effective method to access this class of compound. The dimer, PDI-DPP-DPP-PDI, presented an overall electron-deficient π-conjugated molecular framework with strong optical absorption from 400–800 nm. The low energy absorption from 600–800 nm is a feature not accessible by the monomeric analogue, highlighting the potential of this dimerization method to be used for spectral engineering of π-conjugated materials.


 Please wait while we load your content...
Please wait while we load your content...